Chapter 1:
About Hydrogen Peroxide for Propulsion Applications
• Hydrogen peroxide, H2O2 is a colorless, heavy, strongly oxidizing liquid capable of reacting explosively with combustibles and is used primarily in aqueous solution as a mild antiseptic, a bleaching agent, an oxidizing agent, and a laboratory reagent. In concentrated form -- greater than 80% by mass -- hydrogen peroxide is usable as a liquid oxidizer or mono-propellant for rocket and propulsion systems. These grades are commonly referred to as High Test Peroxide (HTP).
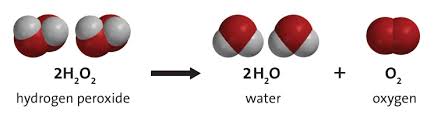
• At room temperatures, HTP can be catalytically decomposed to water and oxygen, along with the release of substantial heat. The associated end-to-end reaction, neglecting intermediate products is
 ...
...
In this reaction both oxidation and reduction occur at the same time ... very energetic decomposition with up to 98 kJ of heat released for every g-mol of hydrogen peroxide that decomposes. This energy release translates to 3.33MJ for every kg of peroxide that is decomposed.
• Typically, in aqueous solution peroxide is reasonably stable requiring an activation energy of approximately 75 kJ/g-mol in the absence of a catalyst.
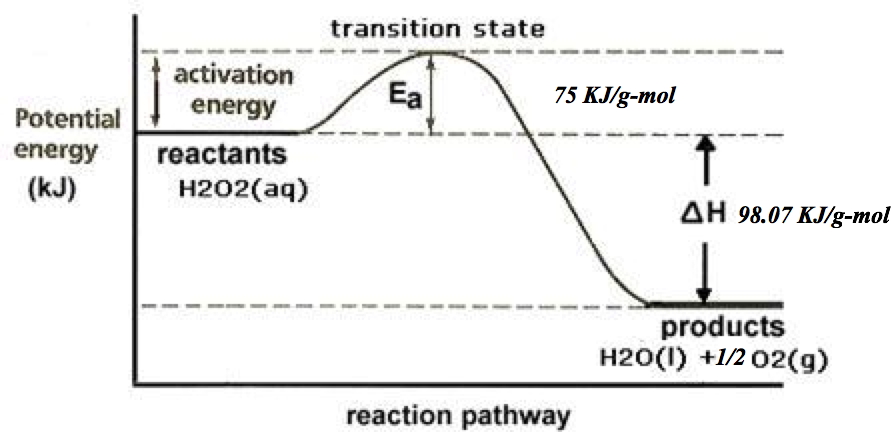
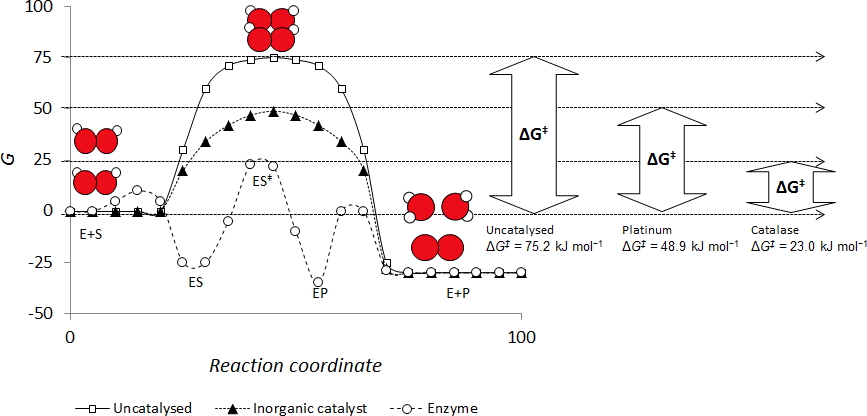
• Noble metal catalysts like platinum or silver can lower the activation energy to less than 50 kJ/g-mol. The organic enzyme Catalase lowers the activation energy to less than 25 kJ/g-mol.
• In typical rocket applications a heated catalyst bed is used to initiate decomposition. The catbed lowers the activation energy to the point where a moderate amount of heat can initiate decomposition.
• Drawbacks to the catalytic decomposition approach include catalyst poisoning due to the presence of stabilizers in HTP, and susceptibility of the metal catalyst to melting because of the intense heat release. This event renders the use of catalysts not only inconvenient but also quite expensive.
• For aqueous solutions with concentrations of peroxide greater than approxomately 67%, sufficient amounts of heat are released to vaporize the warer content and produce steam.
• Most propulsion applications for high-test peroxide (HTP) require mass concentrations of 91% or greater, and as the water content of the aqueous solution increases the performance drop-off is very significant. In concentrated form HTP is classified as an NFPA Class 4 oxidizer, and its use is constrained by very stringent operating, servicing, and storage requirements. In fact considering its low vacuum Isp performance of HTP (185 sec.) when compared to hydrazine (220 seconds), and comparative use restrictions; most applications defer to hydrazine as the superior propellant.
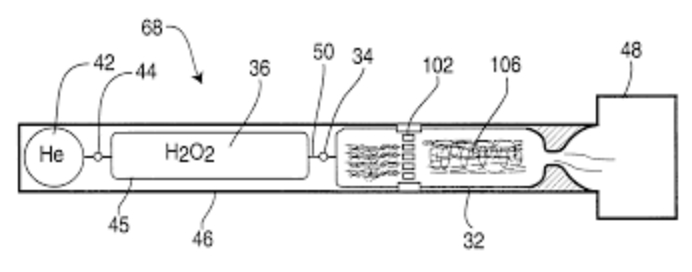
• The proposed research program will use the USU-patented arc-ignition system in conjunction with a catalyst bed to develop a hybrid system that allows lower concentrations of peroxide to provide sufficient heat to sustain a reaction when combined with a fuel source, as in a hybrid rocket.
Research Abstract: Arc-Ignition of a 70% Hydrogen Peroxide/ABS Hybrid Rocket System