Chapter 3:
Hazards Associated with Hydrogen Peroxide for Propulsion Applications

The three main hazards from handling peroxide are
1) Ignition of clothing
2) Chemical Burns on skin, eyes, etc.
3) Injury resulting from rupture of Pressure Vessel of storage container.
• Hydrogen peroxide is a strong oxidizer that has a potential to cause a fire or explosion in contact with incompatible materials such as combustibles (e.g., wood, paper, organic solvents). It is corrosive and light-sensitive. It is toxic if swallowed and corrosive to the eyes and skin. Prolonged exposure may cause dermatitis.
The properties of hydrogen peroxide that contribute to most accidents include :
- Sensitivity to contamination.
- Hydrogen peroxide will decompose if contaminated and the decomposition reaction may be rapid and produce water, oxygen and heat.
- The pressure generated can have deleterious effects on containment systems resulting in explosions that produce shrapnel capable of destroying structures and injuring personnel.
- The heat released from hydrogen peroxide decomposition may be sufficient to initiate combustion of flammable or combustible materials.
- Reactivity with organic materials.
- Hydrogen peroxide will react with a variety of organic materials and can form explosive mixtures, shock sensitive compounds, and initiate fire.
- Reactivity with inorganic materials.
- Hydrogen peroxide will react with a variety of inorganic materials.
- The reaction may be catalytic or may be an oxidation-reduction reaction.
- The reactions with common inorganic materials in bulk, as trace contaminants, or on surfaces typically produce oxygen.
- Reactivity with fuels.
- Hydrogen peroxide will react with a variety of fuels including organic amines, catalytically-enhanced fuels,and hydrocarbon-based fuels.
- This behavior is a primary reason for hydrogen peroxide's use a propellant.
- The reactions may be rapid and are exothermic.
- Exhaust products and propellant residues with some fuels can be toxic to personnel and harmful to the environment.
- Corrosivity to the skin and eyes.
- Hydrogen peroxide may cause chemical burns and blindness upon exposure to the skin and eyes.
- Adequate first aid and medical attention imust be provided in a timely manner.
- Physical hazards.
- Heat, pressure, shrapnel, and fire resulting from explosive and exothermic events can be injurious and sometimes fatal to personnel.
- Decomposition in the body.
- Ingestion and injection of hydrogen peroxide can result in embolisms, damage to internal organs, and other medical complications that, if not treated aggressively, cause severe internal injuries that are sometimes fatal.
Contamination!
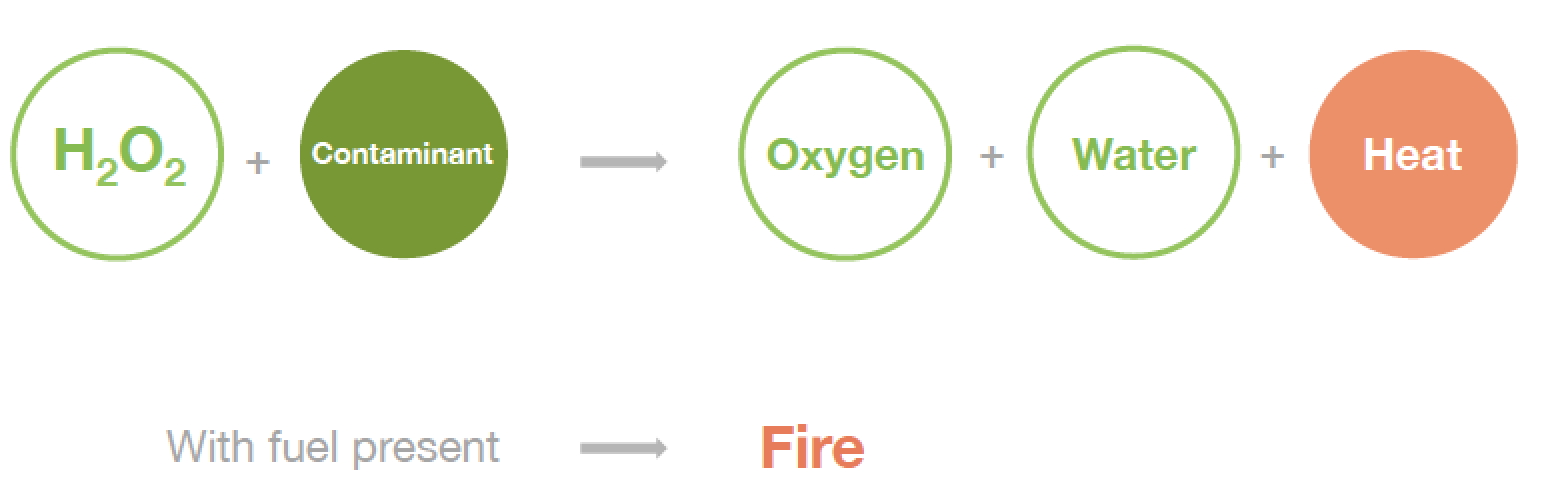
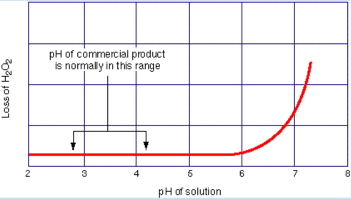
• Contamination by alkaline or organic materials can lower the activation energy much more significantly, sometimes to the point where spontaneous combustion occurs.
•Thus, contamination is the primary risk associated with HTP. Contaminated peroxide can decompose several orders of magnitude faster.

 ...
... 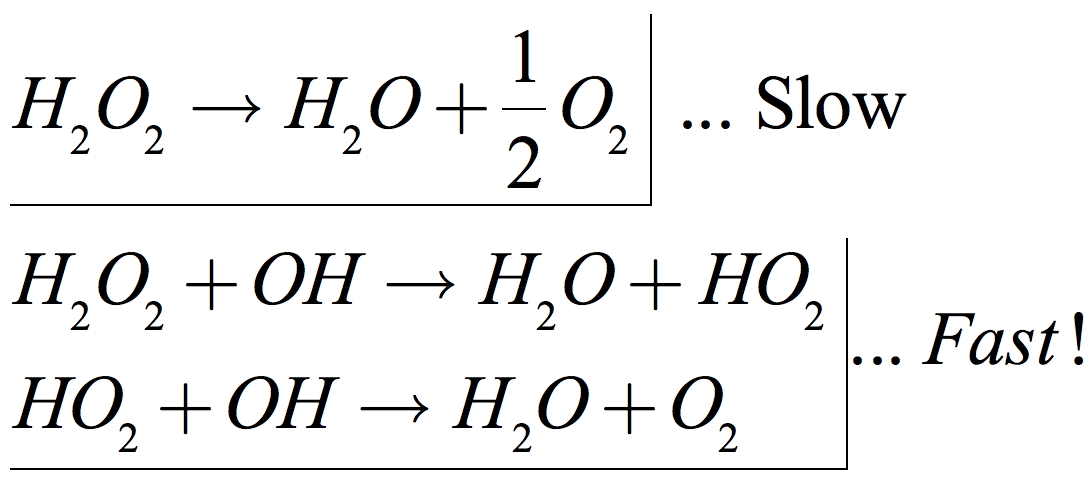
What Sank the Kursk?
 ...
...  ...
... 
Major Causes of All Peroxide Accidents -->
- Failure to recognize hazards
- Failure to follow established procedures
- Improper transportation and storage
- Contaminated hydrogen peroxide or mixtures of hydrogen peroxide with incompatible materials or fuels
- Failure to use appropriate engineering controls or wear appropriate personal protective equipment
- Exposure of the body to skin, eyes, and ingestion
- Injury or death from shrapnel
Evonik Hydrogen Peroxide Safety Training Video