Chapter 6:
H2O2 Passivation Procedures


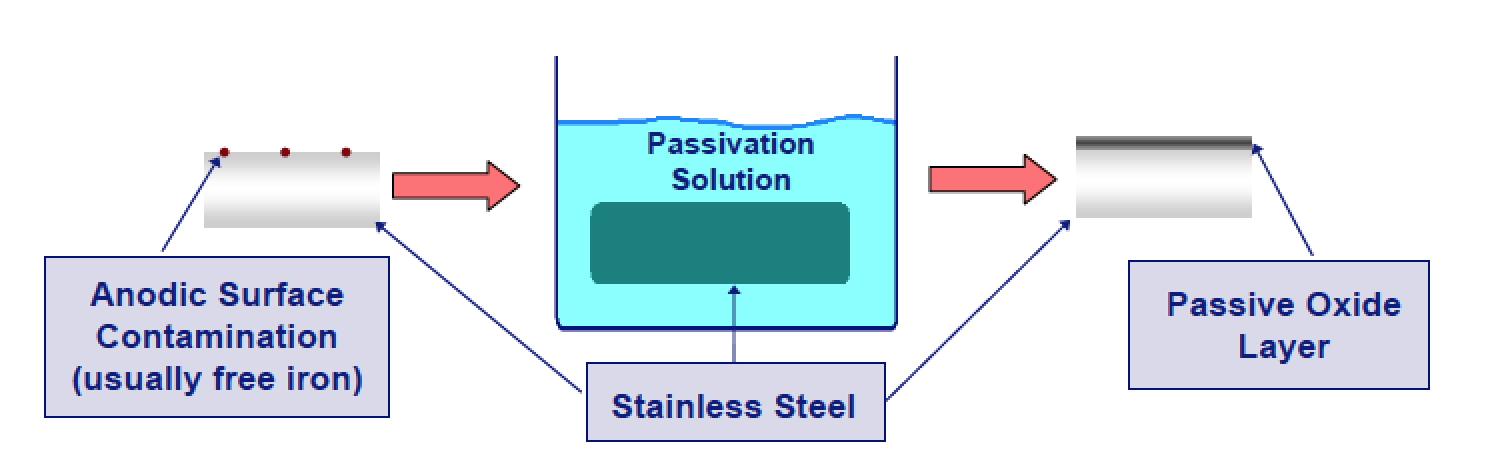
- As a technique, passivation is the use of a light coat of a protective material, such as metal oxide, to create a shell against corrosion.
- Hydrogen peroxide is a strong chemical oxidant which decomposes into water and oxygen in the presence of a catalytic quantity of any transition metal (e.g., iron, copper, nickel, etc.).
- To prevent this unwanted reaction from occurring, any metal surface that comes in contact with hydrogen peroxide must be degreased, pickled and passivated, even if only used once.
- The degreasing and pickling steps chemically clean the metal surfaces.
- The passivating step oxidizes the metal surface.
- The thin oxide coating, which forms on the metal surface during passivation, renders the surface non-reactive to hydrogen peroxide and prevents the metal from decomposing the peroxide.
- Citric Acid Passivation Treatment in Ultrasonic Bath (see link in sidebar)

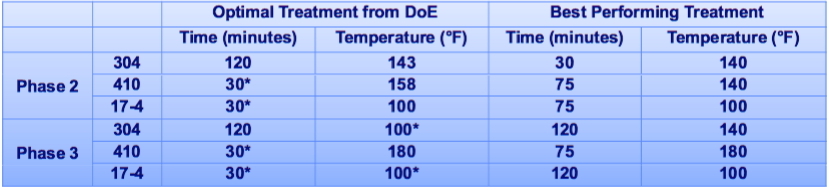
- Use parameters for 304SS since that is closest to the 316SS for test cart.
- Clean items before passivating.
- Passivate using 4% citric acid solution with constant agitation.
- Passivation bath should be at temperatures between 100 degF and 140 degF.
- Leave material in passivation bath for 120 min.