......
......
Chapter 8:
Storage of Hydrogen Peroxide


Containers
- Even at low concentrations, hydrogen peroxide will decompose continuously into water and oxygen.
- This rate is very low when hydrogen peroxide is stored in approved materials and is kept free from contaminants.
- However, if oxygen pressure is not relieved, then high gas pressure may build up.

- Until use, Hydrogen peroxide should be kept in its original container.
- Handling and transferring must be done only with approved dedicated equipment made of compatible material
- Once hydrogen peroxide has been drawn froma storage container, it must not be returned since it may have become contaminated.
- Remember, the primary risk associated with hydrogen peroxide is contamination with organic or akaline materials.


Exposure to Heat
- The decomposition of hydrogen peroxide is exothermic and also the rate of decomposition increases with increasing temperature.
- If the heat of decomposition is not removed at the rate at which it is developed (by heat loss to the surroundings or cooling), the temperature will rise and the rate of decomposition will increase.
- This event can result in a self-accelerating decomposition which, in the case of badly contaminated solutions, may culminate in extremely rapid decomposition or “boil off”.

Venting and Circulation
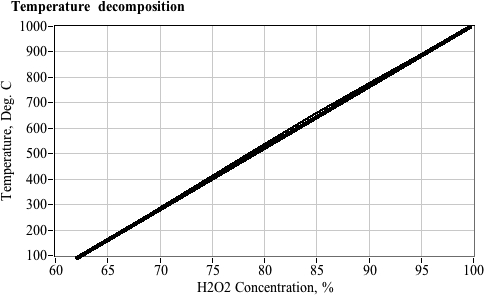
- For solutions up to 64% concentration liquid water is still present, even after the total decomposition.
- Because of this, the final decomposition temperature cannot exceed the boiling point of water.
- For solutions greater than 64% concentration, sufficient heat is generated to evaporate all of the water.
- Super-heated solution has potential to cause container rupture.
- Example, 70% hydrogen peroxide can reach a temperature of 240°C (513 K).
- One volume of 70% solution, when fully decomposed under adiabatic conditions and atmospheric pressure, will
produce about 2,500 volumes of gas compared to the original volume. - Storage site should be chosen so as to avoid contamination and contact with incompatible chemicals, and yet be convenient to the areas of usage.

Storage Container Materials
- Plastic tanks are suitable for up to 50% hydrogen peroxide provided they are made of correct polymeric material.
- Example plastics are polypropylene, polytetrafluoroethylene, polyvinylidene fluoride such as Solvay SOLEF®, and a co-polymer of vinylidene fluoride and hexafluoro propylene such as VITON®.
- Example plastics are polypropylene, polytetrafluoroethylene, polyvinylidene fluoride such as Solvay SOLEF®, and a co-polymer of vinylidene fluoride and hexafluoro propylene such as VITON®.
- White chemical porcelain and borosilicate glass are both compatible with highly concentrated hydrogen peroxide and are widely used for small scale laboratory apparatus.
- Light can cause photochemical decomposition of hydrogen peroxide.
- Amber colored glass contaners extend the lifetime of high concentration H2O2 Solutions.

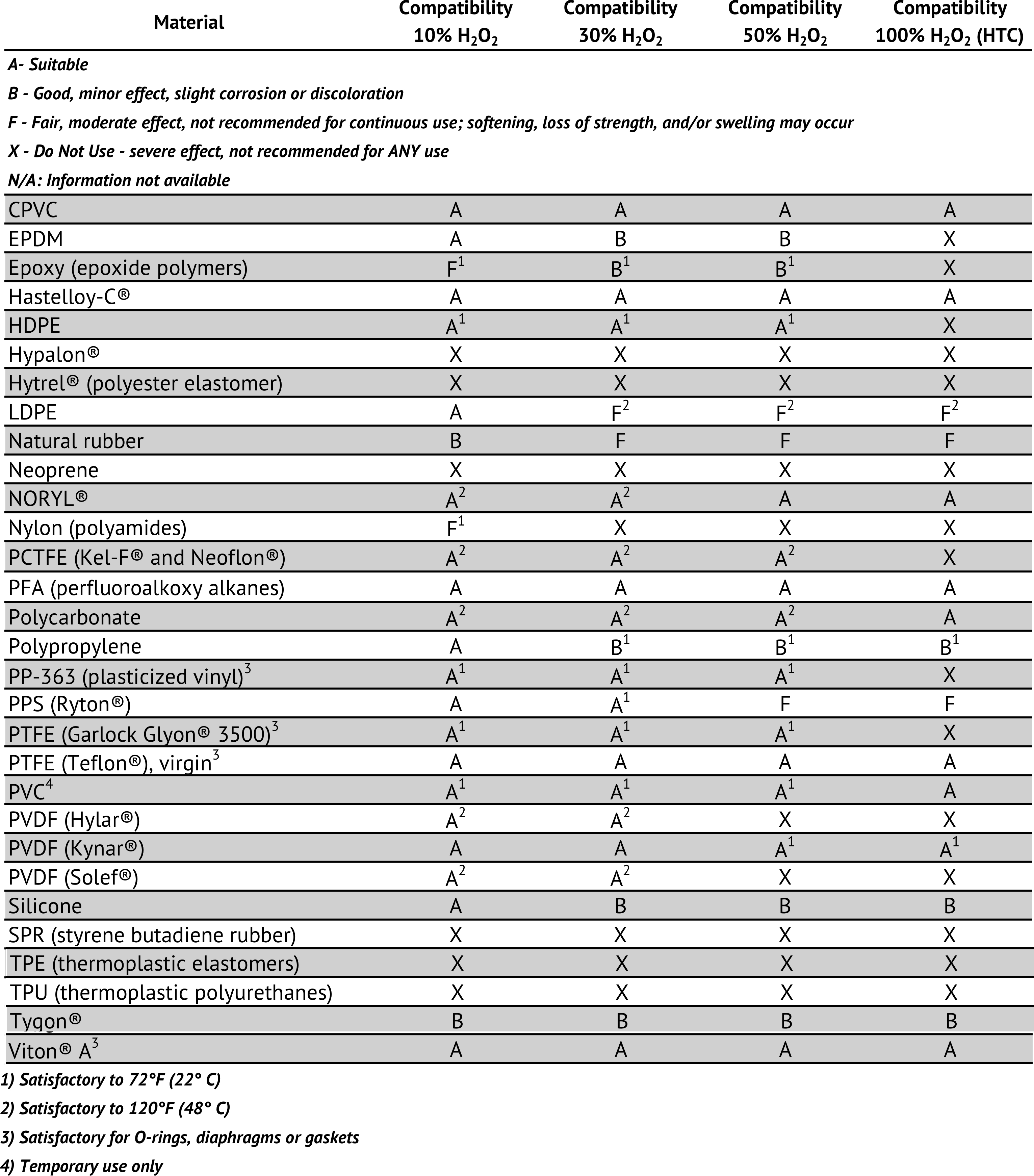
Detailed List of Plastic/Polymeric Materials Compatability